Imagine taking a pill that doesn’t work-again. Or worse, one that makes you sick. For millions of people, this isn’t hypothetical. It’s the reality of trial-and-error prescribing. But what if your doctor could know, before writing the first prescription, whether a drug would help you, hurt you, or do nothing at all? That’s the promise of pharmacogenomics testing.
What Pharmacogenomics Testing Actually Does
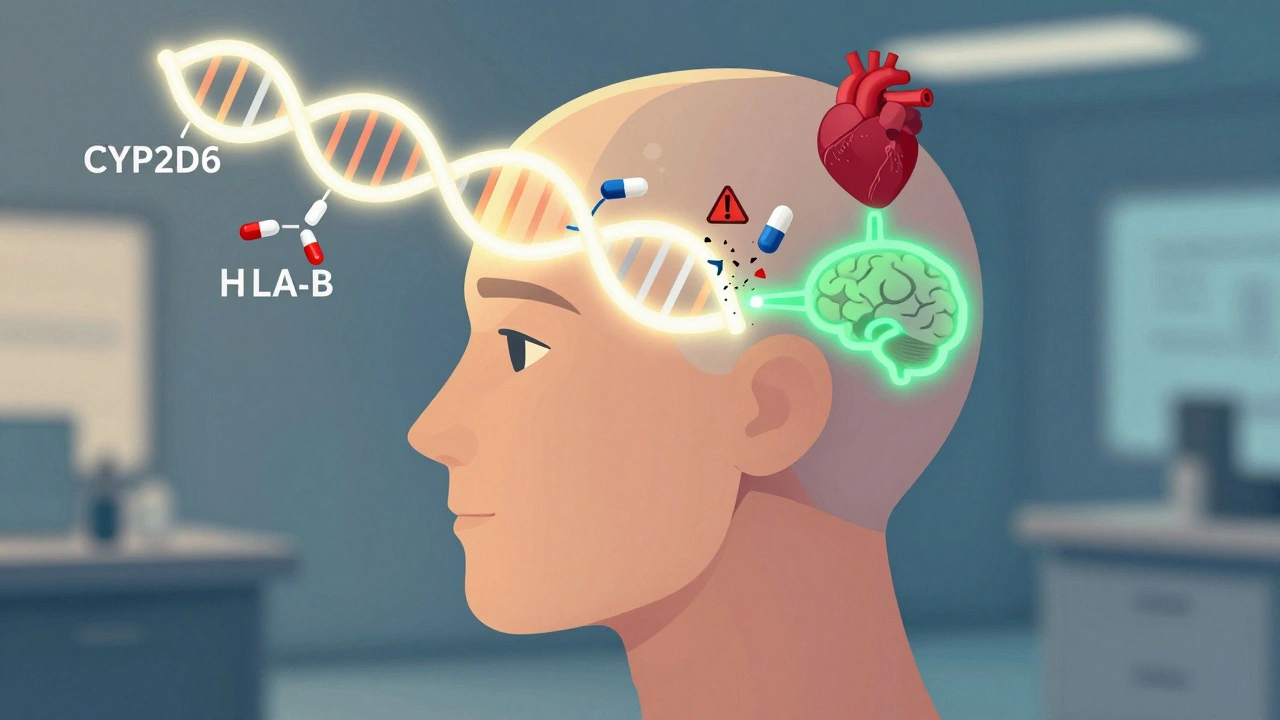
Pharmacogenomics testing looks at your DNA to see how your body processes medications. It’s not about diagnosing disease. It’s about predicting how you’ll respond to a drug. Some people break down antidepressants too slowly. Others clear painkillers too fast. These differences come from tiny variations in your genes, especially in enzymes like CYP2D6, CYP2C19, and CYP2C9. These enzymes handle about 75% of all prescription drugs. A single gene change can turn a lifesaver into a danger zone.The FDA now lists 178 drugs with pharmacogenomic guidance in their labels. One of the clearest examples is abacavir, an HIV medication. If you carry the HLA-B*57:01 gene variant, taking abacavir can trigger a deadly allergic reaction. Testing for this variant before prescribing has nearly eliminated those reactions. That’s not theory-it’s standard practice in clinics today.
Where It Works Best: Real Cases, Real Results
Pharmacogenomics isn’t useful for every drug. It shines where small genetic differences lead to big clinical outcomes. Three areas stand out:- Psychiatry: About 40-60% of people don’t respond to their first antidepressant. A 2022 study in the Journal of Clinical Psychiatry found that patients whose treatment was guided by pharmacogenomics had a 30.5% higher chance of remission compared to those on standard care. One patient on Reddit shared how five SSRIs failed-until a GeneSight test showed she was a CYP2D6 poor metabolizer. Switching to bupropion worked immediately.
- Cardiology: Clopidogrel (Plavix) is a common blood thinner after heart attacks. But if you’re a CYP2C19 poor metabolizer, the drug doesn’t activate in your body. The FDA says these patients have a 50% higher risk of heart events. Testing lets doctors switch to ticagrelor or prasugrel instead.
- Oncology and Pain Management: Tamoxifen, used for breast cancer, needs CYP2D6 to become active. Poor metabolizers get little benefit. For opioids like codeine, the same gene determines if you get pain relief or dangerous side effects from morphine buildup.
For drugs like penicillin or ibuprofen-where dosing is wide and reactions are rare-genetic testing adds little value. But for high-stakes medications, it’s changing outcomes.
How the Test Works and What It Costs
Testing is simple. A saliva swab or blood sample goes to a lab. Most clinical tests focus on a targeted panel of 10-20 key genes, not your whole genome. The most common panels look at CYP2D6, CYP2C19, CYP2C9, SLCO1B1, and HLA-B. Results come back in 3 to 14 days.Costs vary. A targeted panel runs $250-$500. Whole genome sequencing can hit $2,000-but that’s overkill for most patients. Many insurers, including Medicare Part B, cover testing for specific drugs like clopidogrel or abacavir. But coverage for psychiatric panels is spotty: only 35% of private plans pay for it, according to a 2023 American Pharmacists Association survey.
Major labs offering these tests include OneOme, Invitae, and Quest Diagnostics. OneOme’s RightMed test, FDA-cleared in January 2023, analyzes 24 genes for over 350 medications. It’s used in top hospitals like Mayo Clinic and Cleveland Clinic.

Why It’s Not Everywhere Yet
The science is solid. The problem is the system. Only 15% of physicians feel trained to interpret pharmacogenomic reports, per a 2022 JAMA Internal Medicine survey. Most EHRs don’t flag gene-drug interactions automatically. Even when results are in the chart, doctors often miss them.Another issue? The data isn’t complete. Most studies are based on people of European descent. For non-European populations, we’re missing key variants. Dr. Russell A. Altman of Stanford warned in 2023 that over 90% of potential gene-drug links remain uncharacterized in diverse groups. That’s a major equity gap.
And then there’s expectation management. Patients sometimes think the test will solve all their medication problems. But it typically applies to just a few drugs. One patient told Medscape: “It only addressed 3 of my 10 medications.” That’s normal. It’s not a magic bullet-it’s a precision tool.
What’s Changing Right Now
The field is accelerating fast. In 2023, Epic and Cerner-two of the biggest EHR platforms-started auto-flagging high-risk gene-drug interactions. If your chart shows you’re a CYP2D6 poor metabolizer and your doctor tries to prescribe codeine, the system pops up a warning.The NIH’s eMERGE network is testing pre-emptive pharmacogenomics in 100,000 patients across nine health systems. Results are stored in medical records so they can be used anytime, not just once. The All of Us program has already collected genomic data from over 620,000 Americans, with results being returned to participants starting in 2023.
Point-of-care devices are in FDA trials. Imagine a doctor swabbing your cheek during a visit and getting results before you leave the room. That’s coming. By 2027, Gartner predicts 30% of prescriptions will include pharmacogenomic data-up from under 5% today.

Who Should Consider It?
You don’t need to be sick to benefit. But you should consider testing if:- You’ve tried multiple antidepressants without success
- You’ve had a bad reaction to a common drug
- You take five or more medications
- You have a family history of poor drug response
- You’re starting treatment for heart disease, cancer, or chronic pain
It’s not for everyone. If you’re on a simple antibiotic or blood pressure pill that’s working fine, there’s no rush. But if you’re stuck in a cycle of side effects and failed treatments, this could be the turning point.
The Bottom Line
Pharmacogenomics testing isn’t science fiction. It’s here. It’s proven. It’s saving lives in psychiatry, cardiology, and oncology. But it’s not a cure-all. It works best when used with clinical judgment, not instead of it.What’s clear: the future of prescribing isn’t guesswork. It’s genetics. And as costs drop and EHRs get smarter, this won’t be a specialty test-it’ll be routine. By 2030, half of all U.S. adults may have their pharmacogenomic profile stored in their medical record. The question isn’t whether you’ll get tested. It’s when.
Is pharmacogenomics testing covered by insurance?
It depends on the test and your plan. Medicare Part B covers testing for specific drugs like abacavir and clopidogrel. Many private insurers cover it for psychiatric medications if there’s a documented history of failed trials. But coverage for broad panels is inconsistent-only about 35% of commercial plans pay for them. Always check with your insurer before testing.
Can pharmacogenomics testing tell me if I’ll get addicted to opioids?
Not directly. It can’t predict addiction risk. But it can tell you how your body metabolizes opioids like codeine or tramadol. If you’re a CYP2D6 ultra-rapid metabolizer, you may turn codeine into morphine too quickly, leading to dangerous side effects-even at normal doses. If you’re a poor metabolizer, you might get no pain relief at all. This helps avoid ineffective or unsafe prescriptions, but it doesn’t measure addiction potential.
How accurate are pharmacogenomics tests?
The genetic part is highly accurate-over 99% for the variants tested. But predicting how you’ll respond to a drug is more complex. Genes explain only 10-15% of drug response variability for most medications. Age, liver function, other drugs you’re taking, diet, and even gut bacteria also matter. A test doesn’t guarantee success-it gives you the best starting point.
Do I need to retake the test if I get a new one later?
No. Your genes don’t change. Once you’ve had a pharmacogenomics test, the results are lifelong. That’s why pre-emptive testing-doing it once and storing the data in your medical record-is becoming the gold standard. Future doctors can refer back to it anytime you start a new medication.
Can I get tested without a doctor’s order?
Some direct-to-consumer companies offer genetic tests that include pharmacogenomic data. But these aren’t always clinically validated. For medical use-especially if you’re on multiple medications or have a complex history-you need a test ordered by a provider and interpreted by a clinical pharmacist or genetics specialist. Otherwise, you risk misinterpretation or inappropriate changes to your treatment.
Are there risks to getting tested?
The physical risk is zero-it’s just a saliva swab. But there are psychological and privacy risks. Learning you’re a poor metabolizer for a drug you’ve been taking for years can be unsettling. And while GINA protects against genetic discrimination in health insurance, it doesn’t cover life insurance or long-term care. Make sure you understand the privacy policy of the testing company before proceeding.
Ella van Rij
December 3, 2025 AT 15:27Oh wow, finally someone wrote an article that doesn’t sound like a pharmaceutical ad. I mean, who else but a genius would think ‘let’s test genes before giving people drugs’? Groundbreaking. I’m sure my cat’s DNA will be next. 🤡
Alicia Marks
December 4, 2025 AT 05:02This is actually life-changing for so many people. If you’ve ever been stuck on five antidepressants and felt like a lab rat, this is hope. Keep pushing for access - it’s not magic, but it’s real.
Paul Keller
December 5, 2025 AT 16:30While I appreciate the general sentiment behind pharmacogenomics, I must emphasize that the current clinical implementation remains statistically underpowered for population-wide application. The effect sizes for most gene-drug interactions are modest at best, and confounding variables - including epigenetic modulation, polypharmacy, and microbiome variability - substantially dilute predictive accuracy. Furthermore, the economic burden of universal screening is not yet justified by randomized controlled trial outcomes. Until we achieve standardized, interoperable genomic data integration within EHRs, this remains a niche tool for high-risk cohorts.
Shannara Jenkins
December 6, 2025 AT 12:07My mom took 7 different meds for depression before one finally worked - and it was only because her pharmacist noticed her CYP2D6 status from an old test. She cried when she found out it wasn’t her fault. This isn’t futuristic. It’s just… common sense. We need this everywhere.
Elizabeth Grace
December 8, 2025 AT 10:34I got tested last year after my third SSRI failed. Turned out I’m a slow metabolizer. Switched to bupropion and felt like I’d been asleep for 10 years and just woke up. My therapist cried. I cried. My dog cried. We all cried. Thank you for writing this. I finally feel seen.
Steve Enck
December 8, 2025 AT 16:30One must question the epistemological foundations of this paradigm. If our pharmacological fate is encoded in our DNA, are we not merely biological automatons? The illusion of agency in medication choice dissolves under the weight of genetic determinism. Moreover, the commodification of genomic data by corporate labs like OneOme raises profound ethical concerns - are we patients, or are we datasets? The algorithm does not care if you suffer. It only cares if your SNP matches a precomputed risk profile.
Jay Everett
December 9, 2025 AT 22:07Brooooooo. 😎 This is the future. Imagine walking into a clinic, spitting in a tube, and 20 minutes later your doctor says, ‘Hey, don’t take codeine - you’ll turn into a zombie.’ And you’re like, ‘Wait, you knew that? From my spit?!’ YES. YES YOU DID. 🚀 I got tested after my knee surgery - codeine gave me hallucinations, tramadol made me itch like I was on fire. Turned out I’m a CYP2D6 ultra-rapid metabolizer. Now I get tapentadol and I’m not hallucinating or scratching my skin off. This isn’t sci-fi. It’s 2025. Get tested. Your future self will thank you.
Laura Baur
December 11, 2025 AT 18:40It’s amusing how casually people accept genetic testing as a panacea while ignoring the glaring absence of non-European data. You’re telling me that a test developed on 90% white cohorts is being rolled out to Black, Indigenous, and Asian populations as if it’s universally valid? That’s not precision medicine - that’s genetic colonialism. And yet, the same people who scream about equity in education will happily pay $500 for a test that doesn’t even account for their ancestry. The arrogance is breathtaking. Until we fix the data, this is just another tool to widen the health gap - not close it.
Jack Dao
December 13, 2025 AT 05:31Wow. So now we’re genetically profiling people before giving them ibuprofen? Next they’ll test your DNA to see if you’re worthy of coffee. 🤦♂️
dave nevogt
December 13, 2025 AT 20:28I’ve been thinking a lot about how we treat medicine as a one-size-fits-all experiment on human beings. We’ve been doing this for a century - guess, wait, adjust, repeat. And now, finally, we’re beginning to see that bodies aren’t machines with universal parts. They’re ecosystems shaped by evolution, ancestry, and biology. This isn’t just about drugs. It’s about dignity. It’s about not being treated like a statistic. If this helps even one person stop feeling broken because their body didn’t respond the way it ‘should,’ then it’s worth every dollar, every hour, every lab report.
Arun kumar
December 15, 2025 AT 11:42bro this is so cool 😍 i live in india and my cousin took antidepressants for 3 years and nothing worked... i told him to get tested and he did - turns out he's a poor metabolizer. switched to sertraline and now he's back to normal. we don't have this here much but i think it should be free for everyone. genes don't care about money 💪