When you’re on multiple medications that need to be just right - not too much, not too little - you’re dealing with NTI drugs. These are drugs with a razor-thin line between helping you and harming you. Think warfarin, lithium, or levothyroxine. Now imagine taking two of them together. That’s combination NTI therapy. It’s used in serious conditions like heart failure, epilepsy, or certain cancers. But here’s the problem: while single NTI drugs often have generic versions, combination NTI drugs almost never do. And that gap is leaving patients at risk.
What Makes NTI Drugs So Tricky?
NTI stands for Narrow Therapeutic Index. That means the difference between a dose that works and one that causes serious side effects is tiny. For most drugs, a 20% variation in blood levels might not matter. For NTI drugs, it can mean the difference between life and death. The FDA defines them by five key traits: minimal separation between effective and toxic doses, high risk of failure or toxicity from small changes, need for frequent blood monitoring, low variability within a person, and the need for small, precise dose tweaks.
Examples? Warfarin (a blood thinner), levothyroxine (for thyroid issues), lithium (for bipolar disorder), phenytoin (for seizures), and digoxin (for heart rhythm). Each one requires careful monitoring. When you combine two of these - say, warfarin and amiodarone - the margin for error shrinks even further. A small change in one drug can throw off the whole balance. And that’s why generic versions of these combinations are so rare.
Why Aren’t There Generic Combination NTI Drugs?
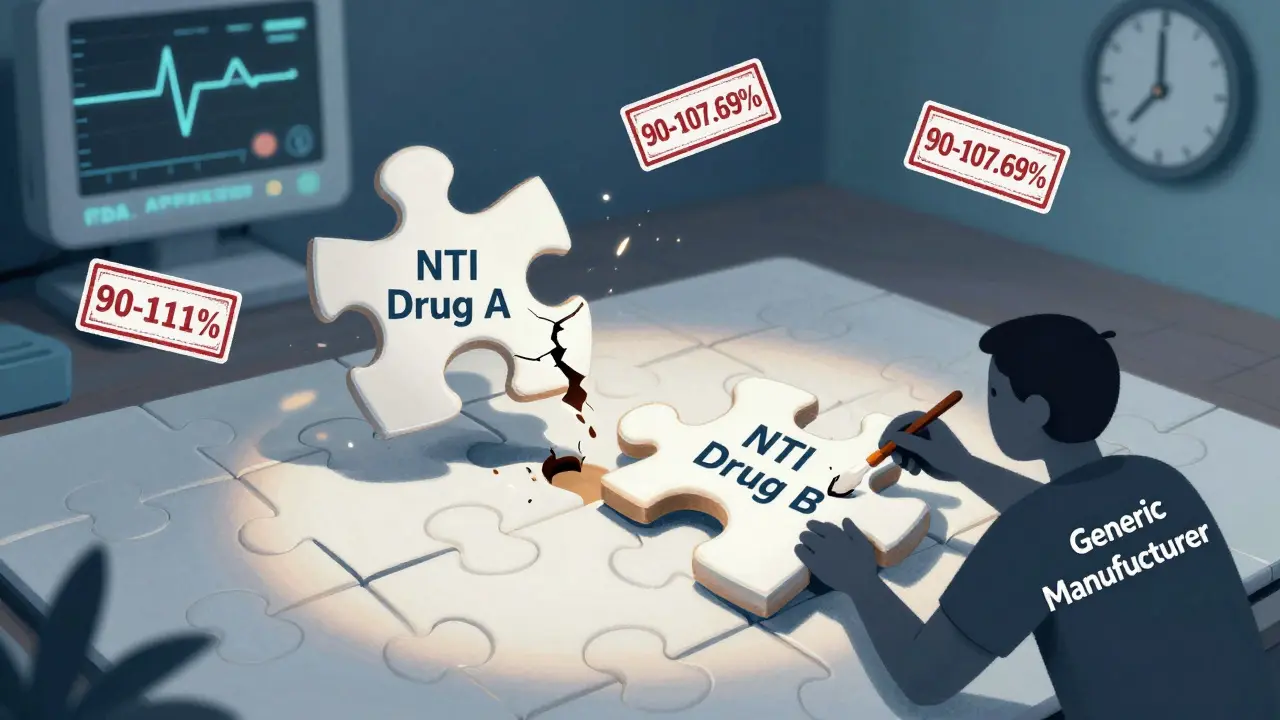
Generic drug makers have to prove their product is bioequivalent to the brand. That means the amount of drug absorbed into your bloodstream has to be nearly identical. For regular drugs, the acceptable range is 80% to 125% of the brand’s levels. For single NTI drugs, that’s tightened to 90% to 111% for peak levels (Cmax) and 90% to 112% for total exposure (AUC). But when you combine two NTI drugs? The math gets worse.
Imagine two drugs, each allowed up to 11% variation. If one is 11% high and the other is 11% low, the combined effect could swing by over 20%. That’s outside safe limits. The FDA’s 2023 draft guidance proposes even tighter standards for combinations: 90% to 107.69% for Cmax and 90% to 110% for AUC. That’s harder to hit than landing a rocket on a moving target.
As a result, while there are 11 approved generic versions of warfarin alone, there are zero approved fixed-dose combinations of warfarin plus another NTI drug in the U.S. The same goes for lithium-carbamazepine combos or phenytoin-levothyroxine mixes. The regulatory bar is so high that no manufacturer has cleared it yet.
The Real-World Cost of the Gap
Patients don’t just lose access to cheaper options - they lose safety. A 2020 JAMA Internal Medicine study found that patients on combination therapy with even one NTI drug had a 27% higher rate of adverse events after switching to generics, compared to 8% for non-NTI combinations. One patient on Reddit shared: “After my pharmacy switched my generic warfarin, my INR went from 2.5 to 6.8 in three days. I ended up in the ER.”
Pharmacists are seeing it too. A 2023 ASHP survey of 856 pharmacists found 78.3% had witnessed therapeutic failure after switching to generic NTI combinations. Over 40% reported serious adverse events - bleeding, seizures, heart arrhythmias. The American Society of Health-System Pharmacists officially opposes automatic substitution of these drugs, calling the risk “unacceptable.”
And it’s not just about side effects. Monitoring costs skyrocket. While non-NTI combinations cost $400-$800 a year in lab tests, combination NTI therapy runs $1,200-$2,500 annually. That’s because patients need weekly or biweekly blood tests for months until their levels stabilize. Some need 3-4 dose changes before things settle. That’s time, money, and stress.
What About Europe? Are They Doing Better?
Some European countries have approved a few combination NTI generics - like levothyroxine plus selenium. Early data shows under 2% adverse event rates. But experts caution: those cases involve drugs with lower inherent risk and more stable pharmacokinetics. The same success hasn’t translated to warfarin or lithium combos.
Even in Europe, the approach is cautious. Dose adjustments are still made individually. Patients rarely get fixed-dose combinations. Instead, they’re prescribed separate generic components. That’s the current workaround in the U.S. too - taking two separate generics. But that’s not the same as a true combination product. It increases pill burden, reduces adherence, and still carries the risk of pharmacy substitutions that change one drug but not the other.
Who’s Affected Most?
It’s not just patients with rare conditions. It’s people managing chronic illnesses with complex regimens. An elderly patient with atrial fibrillation on warfarin and amiodarone. A transplant recipient on tacrolimus and phenytoin. A person with epilepsy on carbamazepine and valproate. These are not theoretical cases - they’re everyday patients. A 2022 Drugs.com survey of 1,247 people on NTI combinations found 63.4% reported adverse effects after generic switches. Only 18.2% of those on brand-name combinations had issues.
And it’s worse in community pharmacies. Enterprise healthcare systems - like VA hospitals or large health networks - are 3.2 times more likely to block automatic substitution than independent pharmacies. Why? They’ve seen the outcomes. They have pharmacists trained in NTI management. Most community pharmacies don’t.
What’s Next? Can This Be Fixed?
The FDA is working on a 2024 pilot program using pharmacometric modeling - computer simulations that predict how drugs behave in the body - to test bioequivalence for combinations. That could be a game-changer. Instead of relying on blood tests in 12-36 healthy volunteers, they might simulate thousands of virtual patients. It’s still experimental, but it’s the most promising path forward.
Manufacturers like Teva and Sandoz argue modern production can meet the standards. But the science says otherwise. As Dr. Donald Berry put it in Nature Reviews Drug Discovery: “A 22% total variation window is fine for one NTI drug. For two? It’s a recipe for disaster.”
Right now, the market for NTI drugs is worth nearly $49 billion globally. But combination NTI products? They make up less than 0.3% of that. That’s not because they’re unimportant. It’s because the science hasn’t caught up to the need.
What Can Patients and Providers Do?
- Ask questions: If you’re on two NTI drugs, ask if they’re brand or generic. If one changed, ask if your levels were checked after the switch.
- Insist on monitoring: Blood tests aren’t optional. They’re your safety net.
- Know your pharmacy: Community pharmacies may switch generics without telling you. Ask for a written record of any changes.
- Push for stability: If your regimen works, don’t let it be changed unless absolutely necessary.
For clinicians, it means learning to manage these regimens like high-risk surgeries - with precision, documentation, and vigilance. Training programs are still rare. Only 12 of 50 major U.S. medical centers have specialized NTI combination clinics. That’s a gap in care.
The truth is, combination NTI therapy saves lives. But without safe, affordable generics, it’s a privilege only some can afford - and only if they’re closely watched. The system isn’t broken because of greed. It’s broken because the science is hard. And until we solve that, patients will keep paying the price.
What are NTI drugs?
NTI drugs, or Narrow Therapeutic Index drugs, are medications where the difference between an effective dose and a toxic dose is very small. Even slight changes in blood levels can lead to treatment failure or serious side effects. Examples include warfarin, lithium, levothyroxine, phenytoin, and digoxin. These drugs require regular blood monitoring and careful dosing.
Why are generic combination NTI drugs so rare?
Generic manufacturers must prove bioequivalence - that their product behaves the same as the brand in the body. For single NTI drugs, regulators require tighter standards (90-111% for Cmax, 90-112% for AUC). But when two NTI drugs are combined, the allowable variation multiplies. A 10% difference in each drug can lead to a 20% total shift in effect - enough to cause toxicity or failure. No manufacturer has yet met the proposed standards for combination products, so none are approved in the U.S.
Can I switch from brand to generic for my combination NTI therapy?
It’s risky. Even if each drug in the combination has a generic version, switching one or both can cause dangerous fluctuations in blood levels. Studies show 27% higher adverse events in combination NTI therapy after generic substitution. Always consult your doctor and pharmacist before any switch. Get your blood tested before and after any change. Never allow automatic substitution without your consent.
How often should I get blood tests if I’m on combination NTI drugs?
When starting or changing a combination NTI regimen, expect weekly or biweekly blood tests for 6-8 weeks. It typically takes 3-4 dose adjustments to stabilize levels. Once stable, testing may drop to monthly or every few months - but never stop. Unlike regular drugs, small changes in diet, other meds, or even sleep can affect your levels. Monitoring isn’t optional - it’s life-saving.
Are there any combination NTI drugs available as generics in the U.S.?
No. As of October 2023, the FDA Orange Book lists zero approved fixed-dose combination products containing two or more NTI drugs in the U.S. While single NTI drugs like warfarin or levothyroxine have multiple generics, combining them into one pill or capsule hasn’t been approved due to unresolved bioequivalence challenges. Patients receive them as separate pills, which increases risk and reduces adherence.
For now, the safest path is to stick with what works, monitor closely, and avoid changes unless under expert supervision. The science is catching up - but until then, patients are left in the gap.
